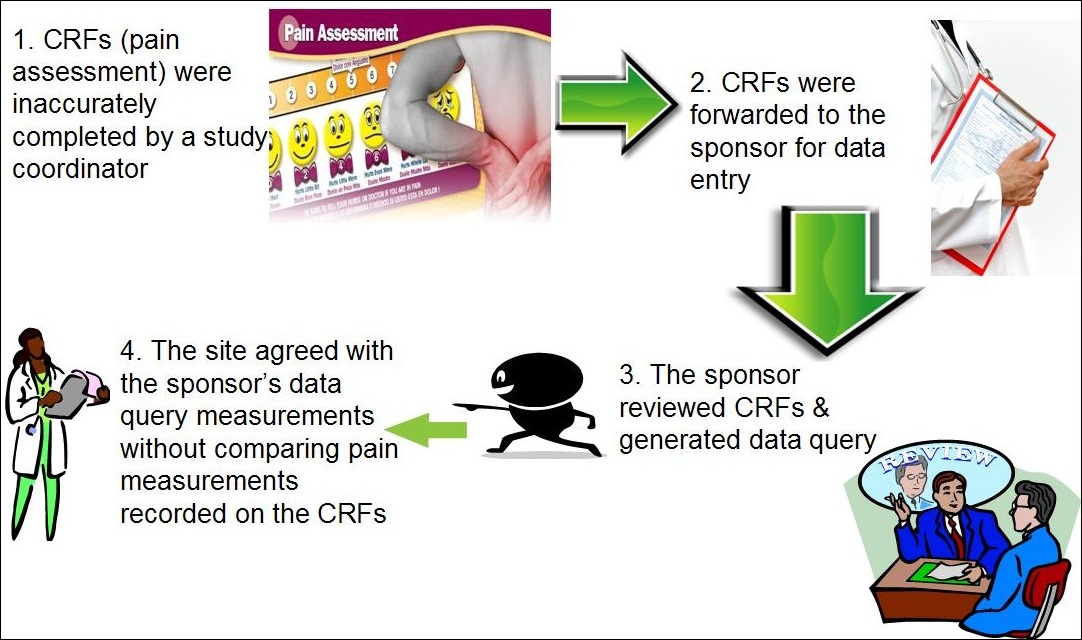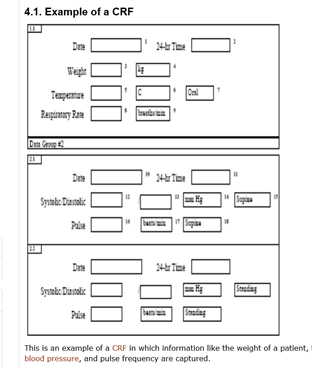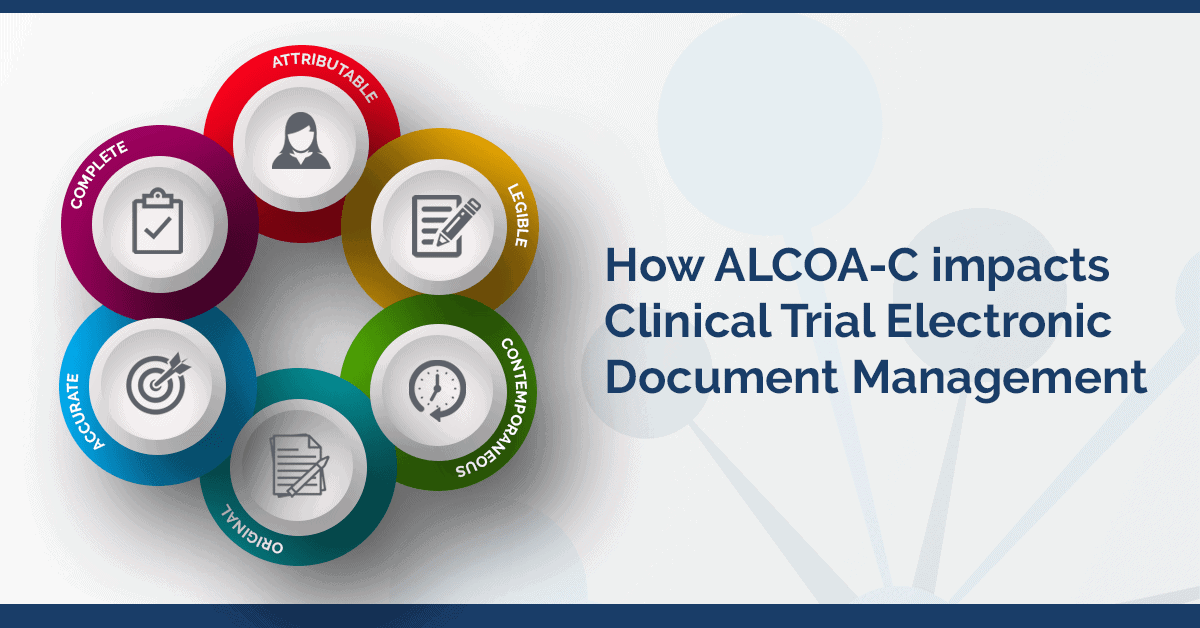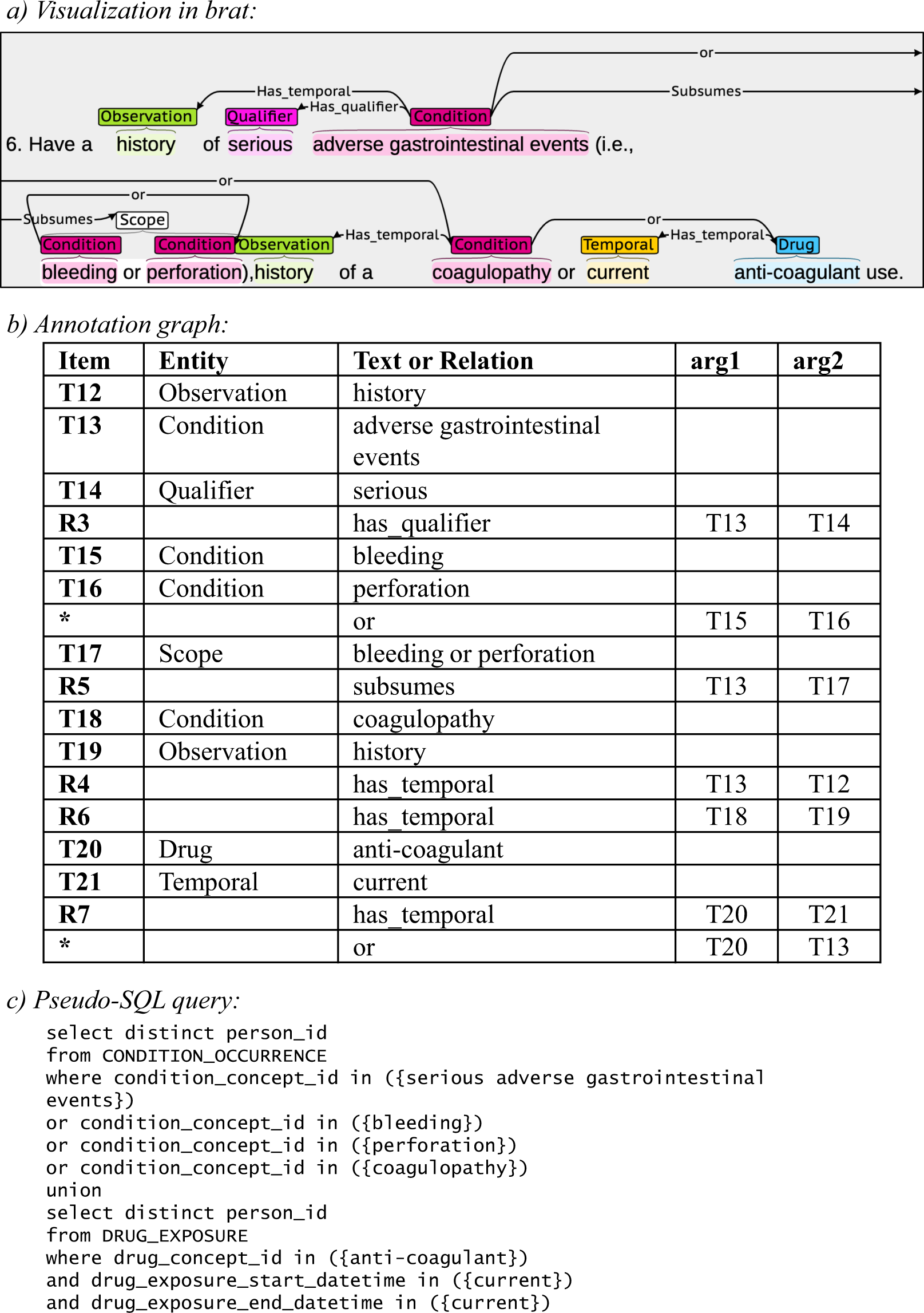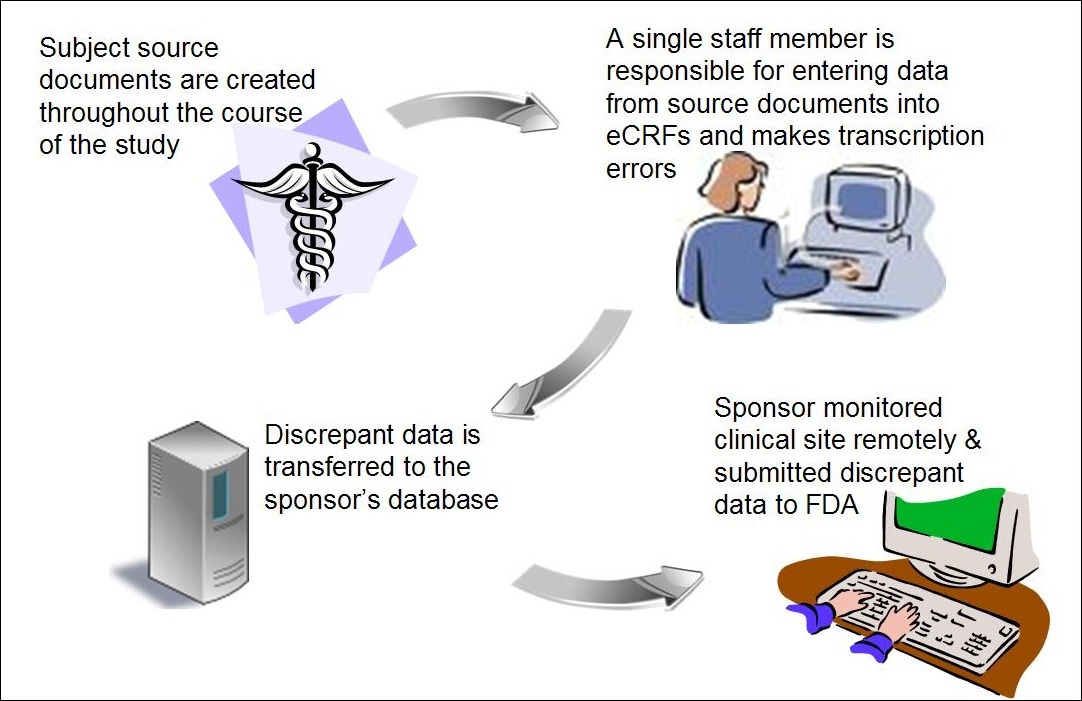
Electronic data capture - Narrowing the gap between clinical and data management – topic of research paper in Clinical medicine. Download scholarly article PDF and read for free on CyberLeninka open science hub.

Electronic Source Data in Clinical Investigations: Navigating the Final FDA Guidance Trailer - YouTube

Data Management for Pharmaceutical Trials Michael A. Kohn, MD, MPP (Acknowledgment: Susanne Prokscha) - ppt download