Principles of Good Clinical Practice (GCP) – What is it all about and who is responsible for adherence? GCP and QA All SIAC Call Mar 14, 2008 Munish Mehra, - ppt download
The EMWA Budapest Working Group: A 2-year collaboration to make recommendations for aligning the ICH E3 guideline with current p

in the united states following the ich e6 guideline is — Clinical Research Certification I Blog - CCRPS
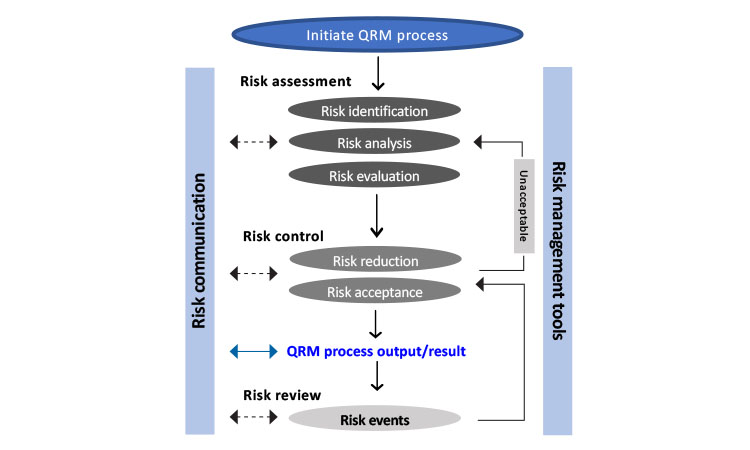
Clinical Trial Management Adaptation to ICH E6 (R2): Good Clinical Practice | Pharmaceutical Engineering
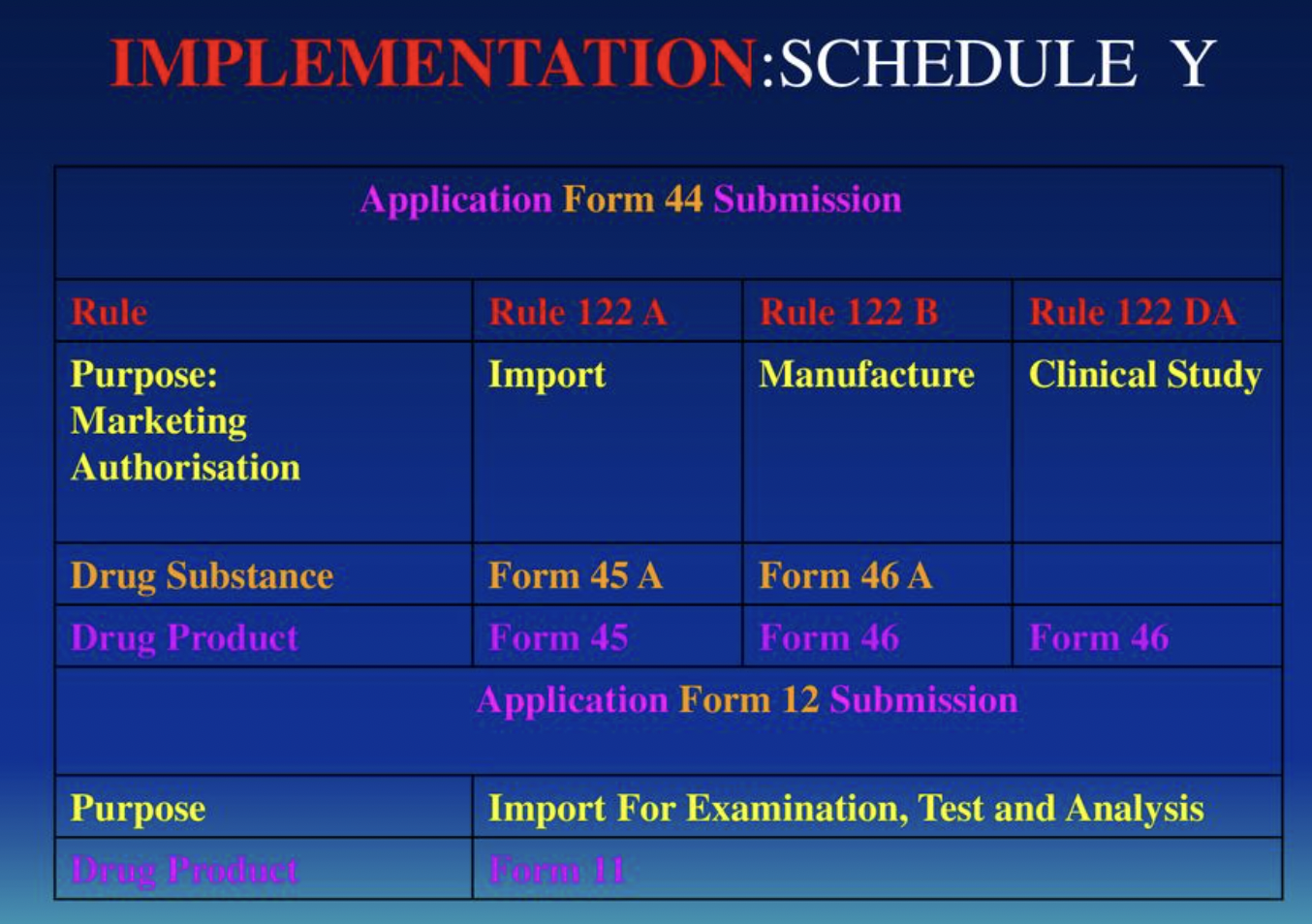
difference between ich gcp indian gcp and schedule y ppt — Clinical Research Certification I Blog - CCRPS

US FDA Guidance – Good Clinical Practice: Integrated Addendum to ICH E6(R1) - Lachman Consultant Services, Inc.

The Impact of ICH GCP E6 Guideline R2 Revisions on Sponsors, Sites, Contract Research Organizations and Vendors | Pharmaceutical Outsourcing - The Journal of Pharmaceutical & Biopharmaceutical Contract Services

Book M1: 2022 Mini Pocket-Sized (3" x 5") ICH Guidelines for GCP (E6) – Clinical Research Resources, LLC