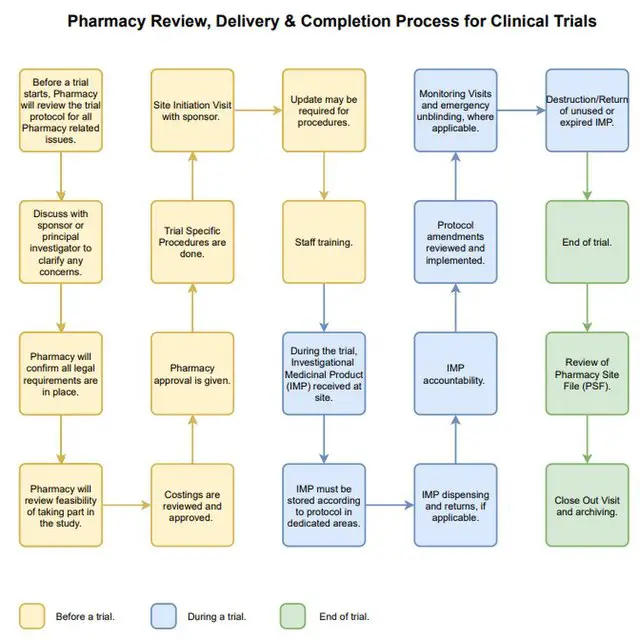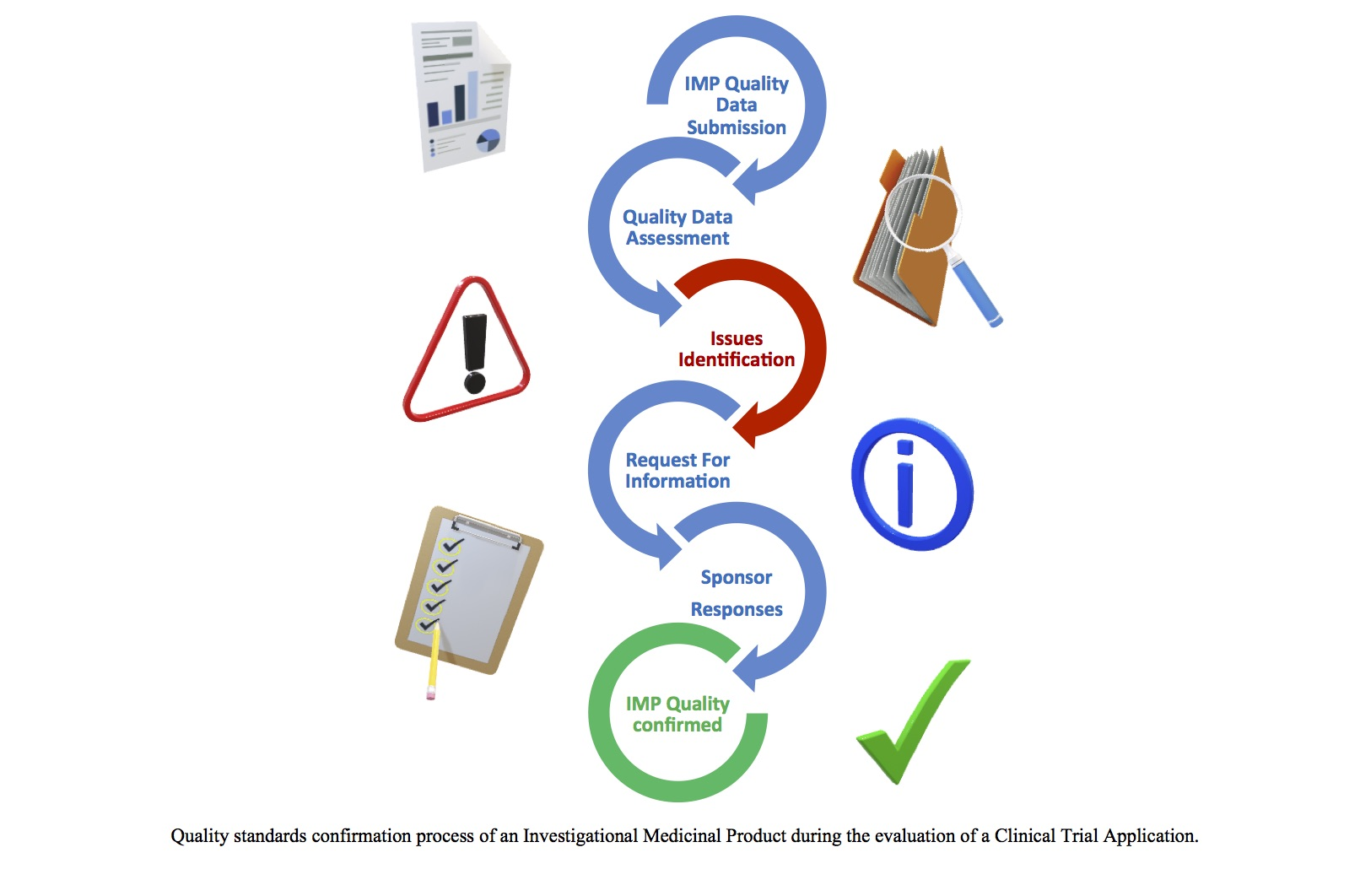
Pharmaceuticals | Free Full-Text | Quality Assessment of Investigational Medicinal Products in COVID-19 Clinical Trials: One Year of Activity at the Clinical Trials Office | HTML
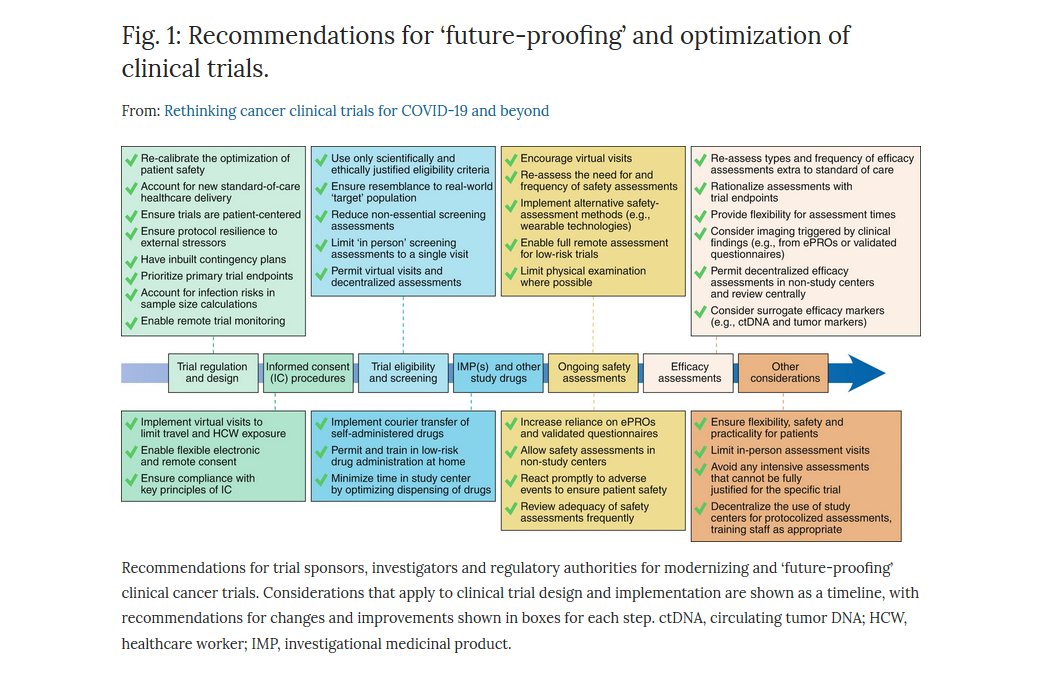
Nature Cancer on Twitter: "ONLINE NOW @NatureCancer: "Rethinking cancer clinical trials for COVID-19 and beyond" by @GaryJDoherty A great discussion about lines of action to ensure the resilience of clinical #cancer research

Proposed clinical trial application dossier. AMPs auxiliary medicinal... | Download Scientific Diagram

Vaccinating Japan: CMIC's efforts to ensure safety and the hope for normalcy - CMIC | Pharmaceutical Development Services (CRO, CDMO, CSO, Healthcare, Japan Entry)