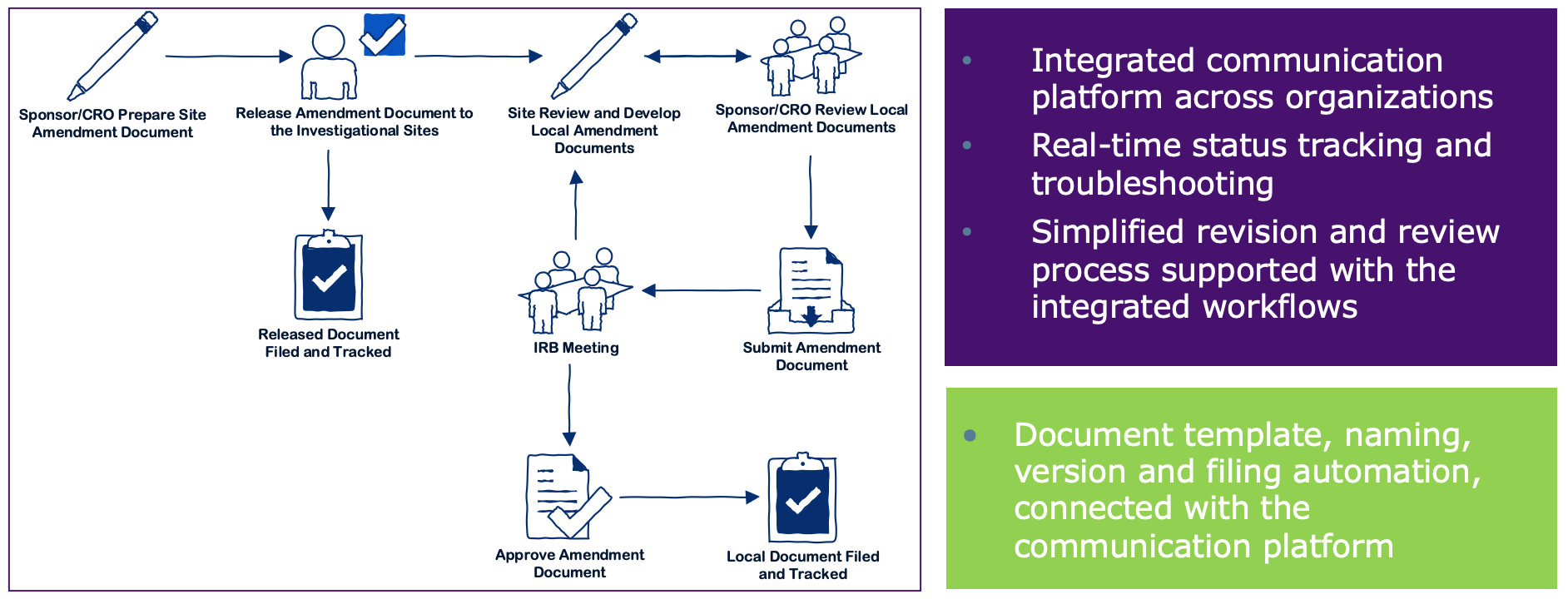
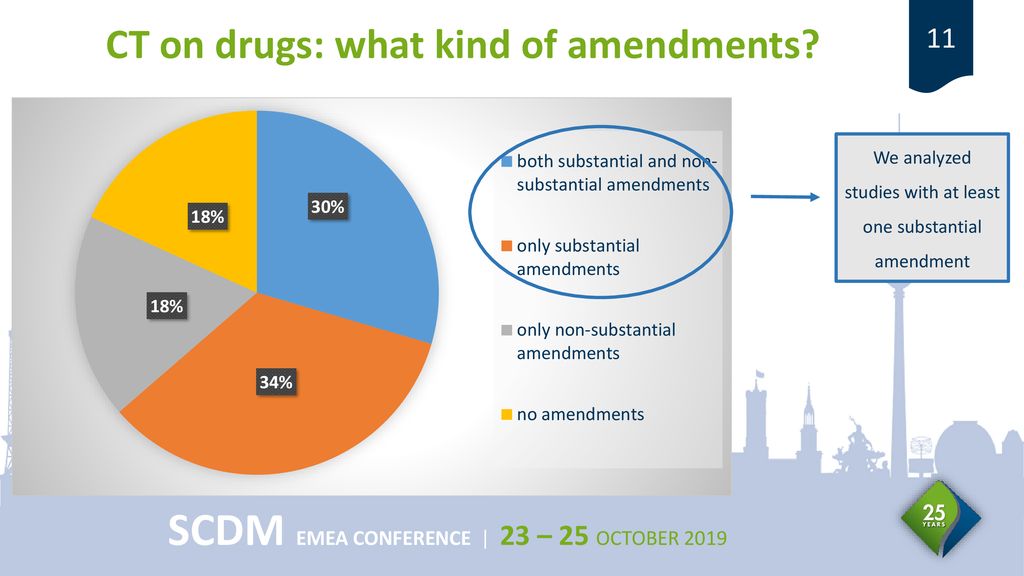
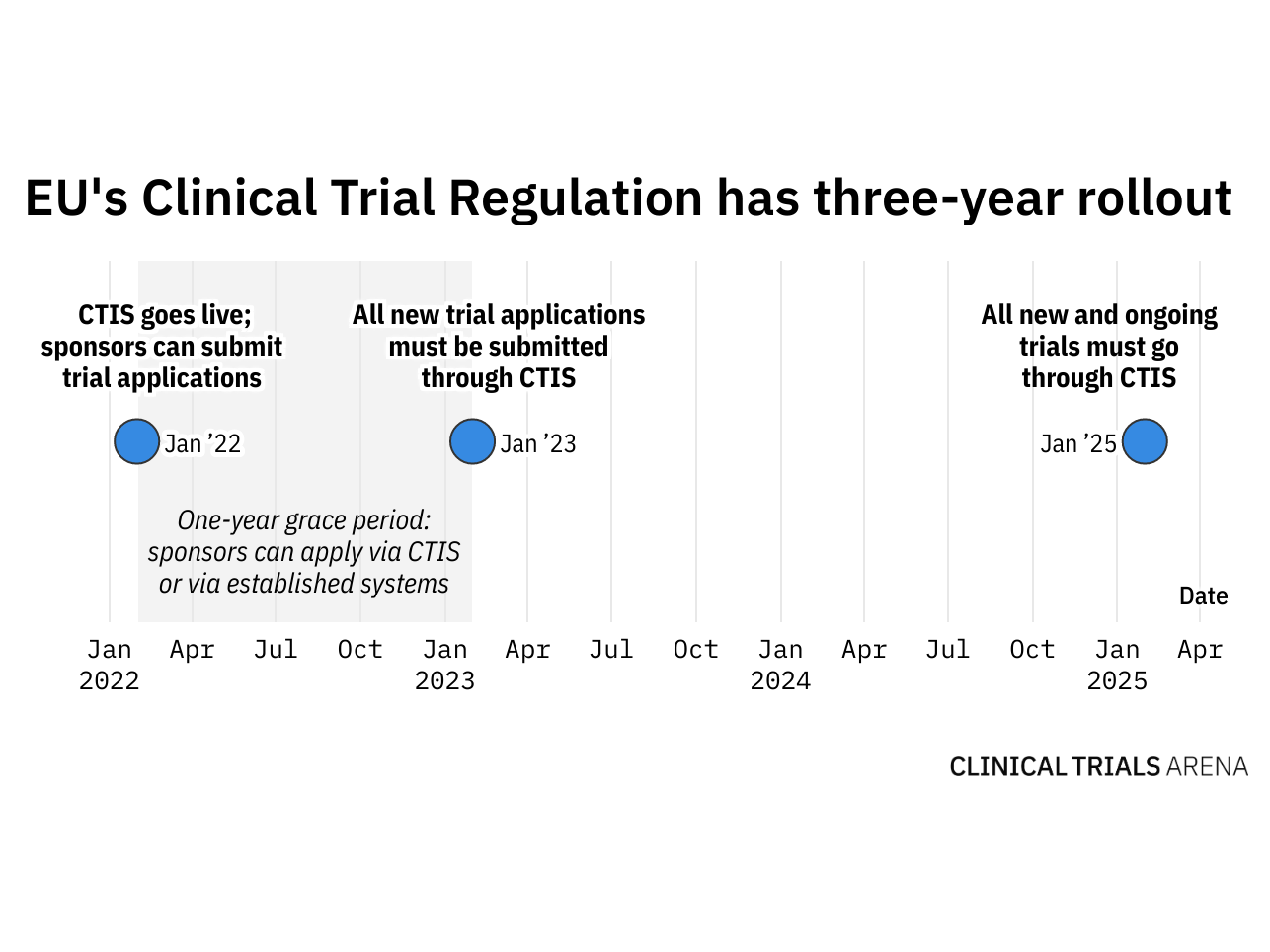
1. Name of service Approval for the substantial amendment (to a clinical trial on a medicinal product for human use) 2. Recipien
Declaration of the End of Trial Form (cf. Section 4.2.1 of the Detailed guidance on the request to the competent authorities for
NOTIFICATION OF A SUBSTANTIAL AMENDMENT TO A CLINICAL TRIAL ON A MEDICINAL PRODUCT FOR HUMAN USE TO THE COMPETENT AUTHORITIES AN
Guide to Clinical Trials Regulation-National Collaboration Project (CTR-NCP) Health Products Regulatory Authority and National