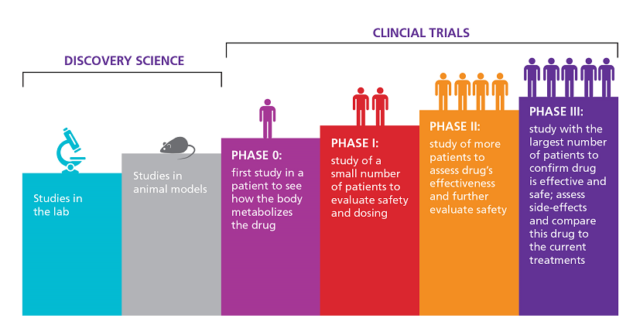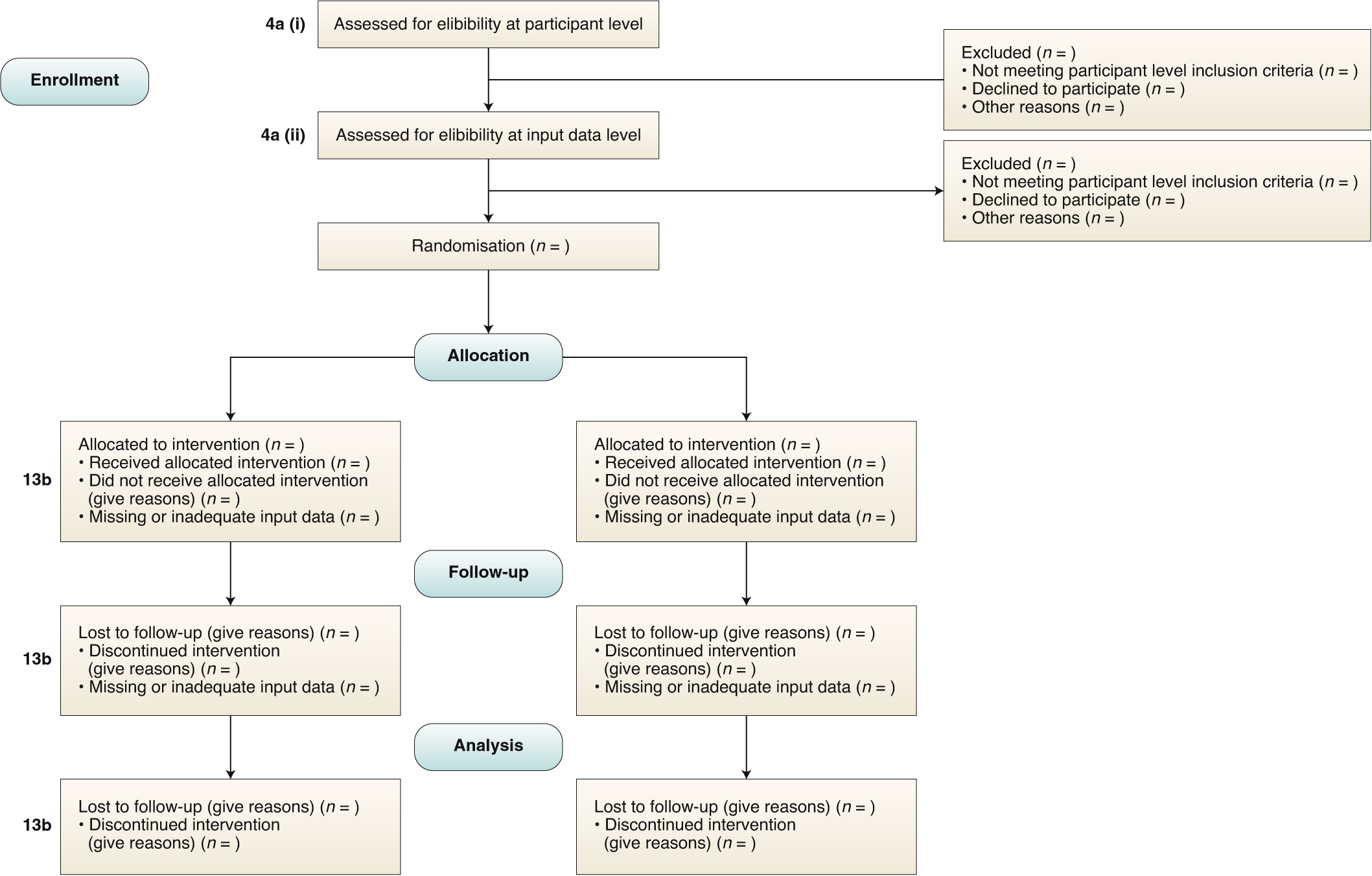
Reporting guidelines for clinical trial reports for interventions involving artificial intelligence: the CONSORT-AI extension | Nature Medicine
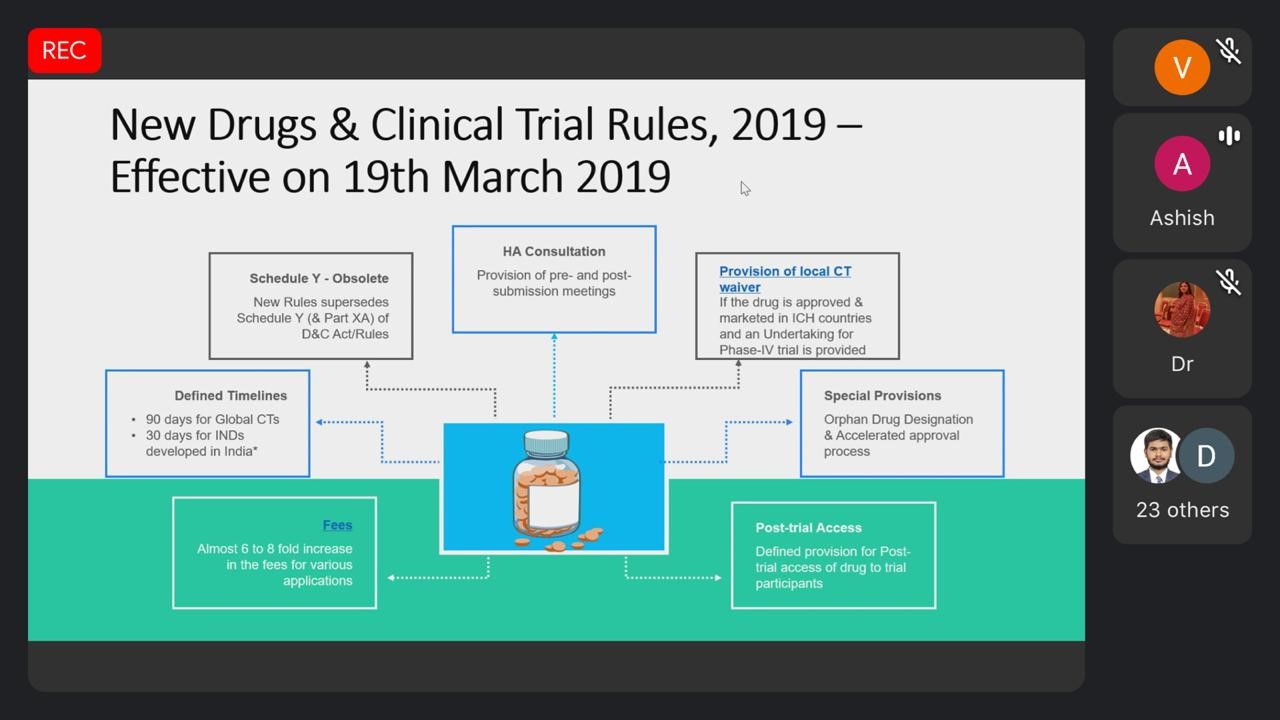
![PDF] New Drugs and Clinical Trials Rules, 2019: Towards Fast-track Accessibility of New Drugs to the Indian Population | Semantic Scholar PDF] New Drugs and Clinical Trials Rules, 2019: Towards Fast-track Accessibility of New Drugs to the Indian Population | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/c3dbb7b610d2b9b9b98495e13eee2bb51f39b8ad/4-Table1-1.png)
PDF] New Drugs and Clinical Trials Rules, 2019: Towards Fast-track Accessibility of New Drugs to the Indian Population | Semantic Scholar

Regulatory Tools ensuring transparency of Registration and Inspection Procedures for entering into the Indian Market Dr. A. Ramkishan M.Pharm, PhD. FIPS, - ppt download
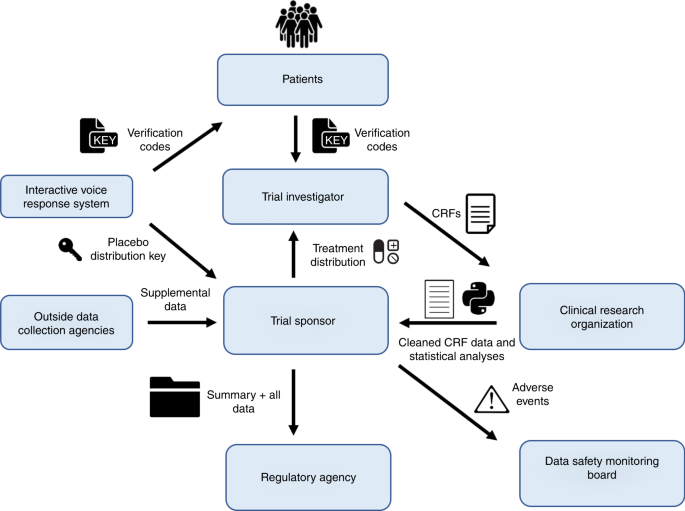
Prototype of running clinical trials in an untrustworthy environment using blockchain | Nature Communications

New Drugs & Clinical Trial Rules, 2019: Buy New Drugs & Clinical Trial Rules, 2019 by Rajan Nijhawan at Low Price in India | Flipkart.com

Uživatel UK House of Commons na Twitteru: „The Medicines for Human Use (Clinical Trials) (Amendment) (EU Exit) Regulations 2019 have been approved by MPs. Find out how it affects post-Brexit arrangements: https://t.co/Foou9RWeqU

New Drugs and Clinical Trials Rules-2019: What academicians need to know - Indian Journal of Dermatology, Venereology and Leprology
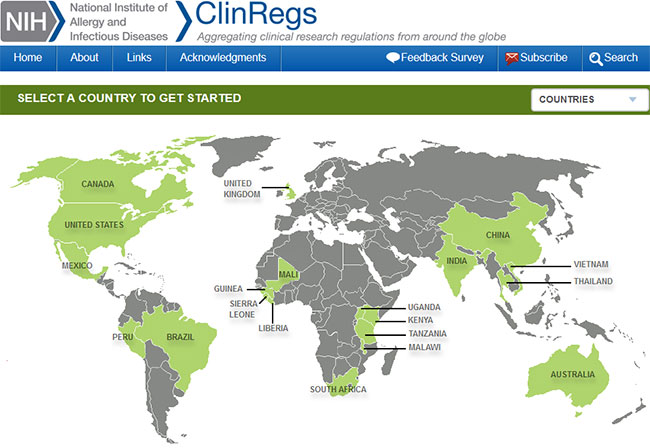
NIH ClinRegs database provides international clinical trial regulations - Fogarty International Center @ NIH
![PDF] New Drugs and Clinical Trials Rules, 2019: Towards Fast-track Accessibility of New Drugs to the Indian Population | Semantic Scholar PDF] New Drugs and Clinical Trials Rules, 2019: Towards Fast-track Accessibility of New Drugs to the Indian Population | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/c3dbb7b610d2b9b9b98495e13eee2bb51f39b8ad/4-Table2-1.png)
PDF] New Drugs and Clinical Trials Rules, 2019: Towards Fast-track Accessibility of New Drugs to the Indian Population | Semantic Scholar

New Drug and Clinical Trial Rules 2019, INDIA What they bring to the table! – Turacoz Healthcare Solutions

The New Drugs And Clinical Trials Rules In India For The Year 2019- ACRI by media.avignaresearch - Issuu